Abstract
Background: The introduction of novel oral immunomodulatory drugs has improved overall survival (OS) rates of patients with multiple myeloma (MM). Poor adherence to oral therapies may lead to drug resistance, poor response to treatment and other untoward effects. The purpose of this study was to examine the impact of socioeconomic status (SES) on adherence to oral MM medications, progression-free survival (PFS) and OS.
Methods: This is a retrospective cohort study from 9/1/18-9/1/21 of data from a large national healthcare payor in the United States. Eligibility criteria include adult patients who had ≥ 2 visits with ICD-10 patient disease codes C90.X, received an oral oncolytic for MM with ≥ 3 fills and had continuous enrollment for six months post-study inclusion. We created SES bands based on the American Community Survey (ACS) and the Behavioral Risk Factor Surveillance System (BRFSS) data and combined "Very Low” and 'Low” and "Very High” and "High” into "Low” and "High” categories, respectively. We measured adherence using the medication possession ratio (MPR), calculated as the total days supplied in the period/length of the period; we defined optimal adherence as an MPR ≥ 0.8. We computed PFS for the entire cohort and defined it as the time from inclusion until the first date of visit with an ICD-10 code of C90.X3 indicting relapse, or date of death, whichever occurred first. We computed OS for the Medicare Advantage (MA) sub-population only (we did not have date of death for the Commercial population) and defined it as the time from inclusion until the date of death, defined as the date of disenrollment with the reason listed as "death.” We used multivariable logistic regression to assess the impact of SES on adherence. We estimated PFS and OS using the Kaplan-Meier method. We used Cox proportional hazard regression analysis and Aalen's additive hazard model to determine which variables correlated with PFS and OS; p-values less than 0.05 were significant.
Results: 1,351 patients met the inclusion criteria; most 755 (55.9%) were adherent. The mean (SD) age was 71.5 (10.4) years, 736 (54.5%%) were men, 779 (57.7%) were White, 656 (48.6%) had a low SES and 972 (77.6%) had MA coverage. The median National Cancer Institute (NCI) comorbidity index was 0.47 (Interquartile range (IQR) 0 to 1.03). There were high rates of polypharmacy, with 29.2% of the population receiving 5-7 different classes of therapies.
In the multivariate model, age was the only factor significantly associated with increased odds of adherence. Patients between the age of 65 to 73 years and 74 to 79 years of age were significantly more likely to be adherent to oral MM agents 1.55 (95% CI, 1.12-2.14) (p< 0.001) and 1.49 (95% CI, 1.05-2.11) (p< 0.001), respectively when compared to patients < 65 years of age. Patients with a low SES, compared with a high SES, were less likely to be adherent 0.77 (95% CI, 0.57-1.03); however, this association was not statistically significant.
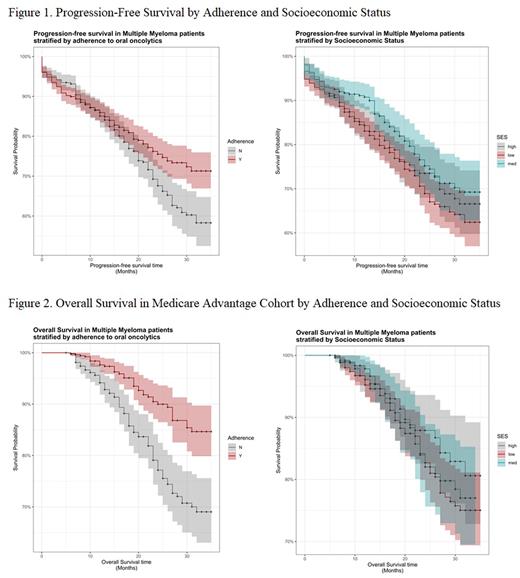
When examining PFS by adherence, significantly more patients experienced progression in the non-adherent cohort (25.7% vs. 19.9%; p = 0.13) compared to the adherent cohort. The proportion of patients who did not progress to disease at 36 months was 71.3% in adherent patients and 58.3% in non-adherent patients (p=0.03) (Figure 1). Adherence was significantly associated with a lower hazard of progression, controlling for SES, NCI score and being a new MM patient, p = 0.016. There was no significant association between PFS and SES.
OS was calculated for the MA subpopulation only. Death was significantly less likely to occur in the adherent cohort (7.0% vs. 17.8%; p < 0.001) compared to the non-adherent cohort. The proportion of patients still alive at 36 months was 84.7% in adherent patients and 69.0% in non-adherent patients (p<0.01) (Figure 2). Adherence was associated with a lower hazard of death, controlling for SES, NCI score and age, p <0.01. There was no significant association between OS and SES.
Conclusions: SES was not associated with adherence, PFS or OS in this study. However, adherent patients experienced significantly longer PFS and OS. To the authors’ knowledge, this is the first study evaluating drug adherence in MM oral agents across the United States. Future studies are needed to determine risk factors influencing non-adherence to oral MM agents so that vulnerable patients are identified and corrective actions are taken to maximize adherence and increase therapeutic success.
Disclosures
Rutter:CVS Health: Current Employment. Avalos-Reyes:CVS Health: Current Employment, Current holder of stock options in a privately-held company; Moderna: Current holder of stock options in a privately-held company; Pfizer: Current holder of stock options in a privately-held company; Haleon PLC: Current holder of stock options in a privately-held company; Astrazeneca PLC: Current holder of stock options in a privately-held company; Johnson & Johnson: Current holder of stock options in a privately-held company; Glaxosmithkline PLC: Current holder of stock options in a privately-held company; Viatris Inc: Current holder of stock options in a privately-held company; Novavax Inc: Current holder of stock options in a privately-held company. Cavers:CVS Health: Current Employment, Current holder of stock options in a privately-held company, Research Funding. Prakash:CVS Health: Current Employment, Current holder of stock options in a privately-held company. Johnson:CVS Health: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: IQVia. Bernal-Mizrachi:Winn Career Development Program: Other: Scientific Board; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kodikaz Therapeutic Solutions LLC: Other: Founder and Scientific Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal